Table of Contents
This is an old revision of the document!
3. Light and Telescopes
1. Light
Light can be described both as a particle and a wave. Light is made of particles called photons and waves called electromagnetic waves. Let us try to understand light as a wave first.
The best way to visualize a wave is to use the example of a water wave. In the following video, you see the largest replica of an ocean created inside a huge dome by US Navy. They use this indoor ocean the size of a football field for experimenting with the effect of waves on oceangoing ships.
If you see the first five minutes of the video, you will realize that waves are created by huge ‘oscillators’ on the sides of the pool. Compare this video with your experience of watching waves in a river, sea or just in a bucket of water. If you stir the water in a bucket with your finger, ripples are created on the surface of the water that travel in different directions. When a boat passes through the center of a river, you see waves coming toward you standing on the bank. If you have been to a sea beach, you have felt the power of such a wave, the amount of energy such a wave can carry.
To create a wave, you must have a medium and a mover that can create motion in the medium. In case of a river, water is the medium and a boat could be the mover. In case of the ocean, mover is the moon, and your finger is the mover in case of a bucket of water.
What are the medium and mover for producing light waves? Charged particles are the mover and the electromagnetic field is the medium. The most familiar charged particle is an electron (responsible for electricity). If electrons move with an accelerated motion in an electromagnetic field, waves are produced in the field and light is nothing but this ‘electromagnetic’ wave. Remember, the field itself is not light, only the wave in the field is light.
Lightning is an enlightening example (video shows the famous lightning in the film Back to the Future from the 1980s). During the terrifying summer monsoon of Bangladesh in Barsha, you see lightning and thunder regularly for almost two months. They are more spectacular at night. How are these light and sound produced? When rain-clouds heavy with water (called ‘Pushkara’ by Kalidasa) gather in the sky, some parts of a cloud could become ‘positively charged’ and some other parts ‘negatively charged’. These ‘positive’ and ‘negative’ poles are nothing mysterious, negative parts have more electrons (which are negative) than the positive parts, that is all. The diagram below shows such a configuration.
Normally electrons cannot jump through air in an empty space. But when the difference between the positive and negative parts becomes too large, electrons rush toward the positive parts breaking the barrier of air. During this ‘discharge’ of high-energy electrons, light (lightning) and sound (thunder) are produced. Light is produced because the electrons stir the electromagnetic field creating ripples. And sound is produced because the heat released by the electrons expand the surrounding air violently producing something like an explosion, this is why thunders sound like an explosion. Imagine a gas cylinder suddenly exploding because of extreme heat; a thunder is very similar.
So light is nothing but waves. A wave can be completely described by its amplitude and frequency. Imagine you are standing on a sea beach knee-deep in water. You will feel one wave after another hitting you. Some waves are higher than others and ‘amplitude’ is nothing but the height of the wave. On the other hand, sometimes one wave comes very soon after another, sometimes they come slowly. Frequency is a measure of how closely spaced the waves are. High frequency waves would come to you literally more frequently (ignoring the ‘wave speed’ for now).

The top of the wave is called ‘crest’, the bottom the ‘trough’ (ট্রফ) and the distance from one crest to another is called ‘wavelength’ which is related to the frequency. Wavelength is the inverse of frequency. A high frequency wave has shorter wavelength, a low frequency wave has longer wavelength.
You can see the relationship between wavelength and frequency in the above simulation. Change the frequency using the slider and see what happens to the wavelength. You can also vary the amplitude and time. Varying the time would make the wave travel horizontally right or left.
Light can also be thought of as made of particles called photons and the energy of these photons are directly related to the frequency mentioned above. High-frequency light waves are made of high-energy photons, low-frequency light waves are made of low-energy photons.
2. Color
It turns out ‘color’ is nothing but the ‘wavelength’ or ‘frequency’, each color is a light of a specific wavelength. There is only wavelength corresponding to each frequency. The wavelengths corresponding to the different colors of visible light are shown below.

The wavelength here is given in nano-meters (nm). So the shades of violet have wavelengths between 380 nm and 450 nm. Shades of blue have wavelengths between 450 nm and 495 nm. The range for green is 495–570 nm, for yellow 570–590 nm, for orange 590–620 nm and, finally, the wavelengths for giving the shades of red vary from 620 nm to 750 nm. Notice that the ultraviolet (to the left of violet) and infrared (to the right of red) parts are blackish because we cannot see those colors. Ultraviolet radiation from the sun is harmful for us and our body radiates infrared light to get rid of our excess energy as heat.
Check the following GIF animation to get the relative frequency corresponding to these wavelengths.
Sunlight is originally white. Newton passed this white light through a prism and understood that the white color is actually made of the 7 colors of the rainbow as shown here. You can clearly see that the violet waves have shorter wavelength and higher frequency compared to red waves. The other colors have wavelengths and frequency that vary between those of violet and red.
One way you can feel closer to the concept of color is via clothes. In the classroom students are wearing clothes of different colors. Let us say the teacher brings to the front seven students who are wearing clothes of seven different colors of the rainbow: violet, indigo (dark blue), blue, green, yellow, orange and red. You can see the clothes because light reflected from them is reaching your eyes. But why do they have different colors.
They have different colors because each of their clothes is reflecting the light of a different color or wavelength or frequency. Red garments have special ‘red’ pigments that absorb all colors of white sunlight except the red. They look red because they can reflect red light very efficiently. Violet clothes reflect violet light and so on.
Why are we studying colors in astronomy? Because astronomical objects (stars, planets, nebulas, galaxies) also emit light of different colors and we can learn a lot about them by analyzing their colors. Compare this with a movie. A movie is made using light. Light of different colors reflected from the clothes and bodies of the actors and from the surrounding are captured using a movie camera and ‘motion pictures’ are created from the captured light. When you see the reflected light on your screen you understand the characters; just from the images and colors you can understand the feelings and inner thoughts of the characters in a movie.
In astronomy, we can know the inner structure of stars and galaxies using their light of different colors detected by our telescope. And not just that, we can also know something about the chemical composition of stars and galaxies using colors.

This is the ‘spectrum’ of sunlight, meaning the light of the sun at different colors. Note that this spectrum is not the same as the spectrum you saw in the beginning of this section. The previous spectrum was smooth, that did not have any black lines and showed all the shades of all the seven colors. But some shades of some of the colors are missing in this spectrum. There are hundreds of such ‘missing colors’ and only a few prominent missing colors (called ‘absorption lines’) have been identified here using the letters A, B, C, D, E, F, G, H and K.
Why are some colors (light at some wavelengths or frequencies) missing? The sun emits smooth light, meaning it emits light at all colors from violet to red. But the sun has an atmosphere where there are many different chemical elements. The elements absorb light of specific colors. Each element can only absorb the light of one specific color. Each line correspond to the absorption of light at a specific color by a specific element. So the lines directly tell us which elements are present in the atmosphere of the sun.
A and B lines are created by oxygen molecules, C lines by hydrogen atoms, D by sodium, E by iron, and G, H and K by calcium. So the dark ‘absorption lines’ or the ‘missing colors’ give away the chemical composition of stars.
3. Telescopes



4. eVscope and eQuinox
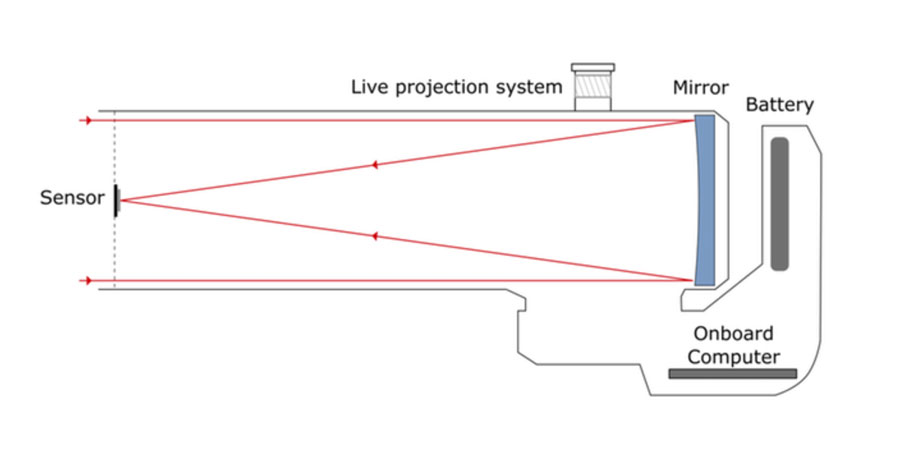
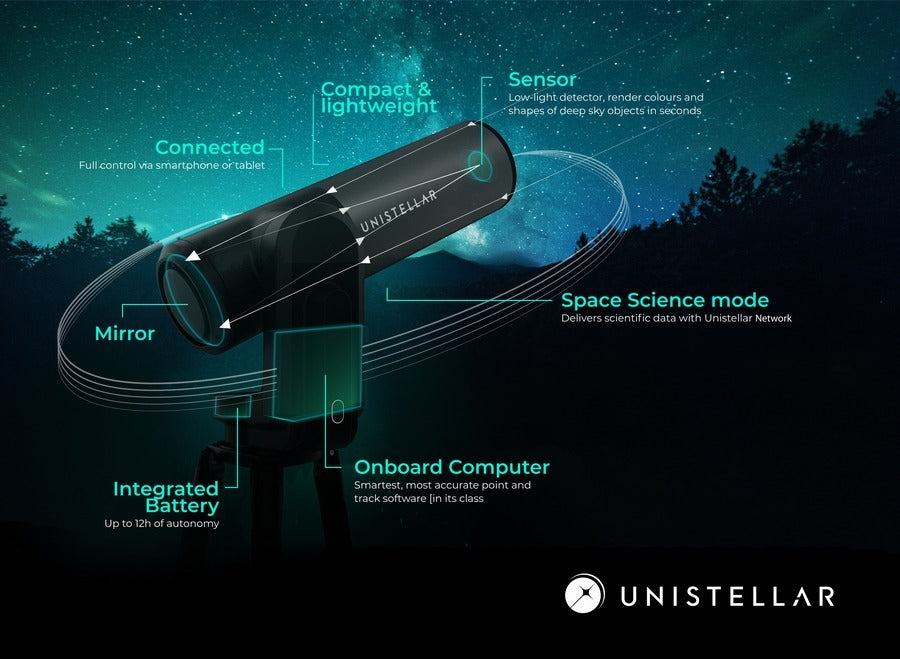
5. Seeing the invisible



