Table of Contents
8. Second law of thermodynamics
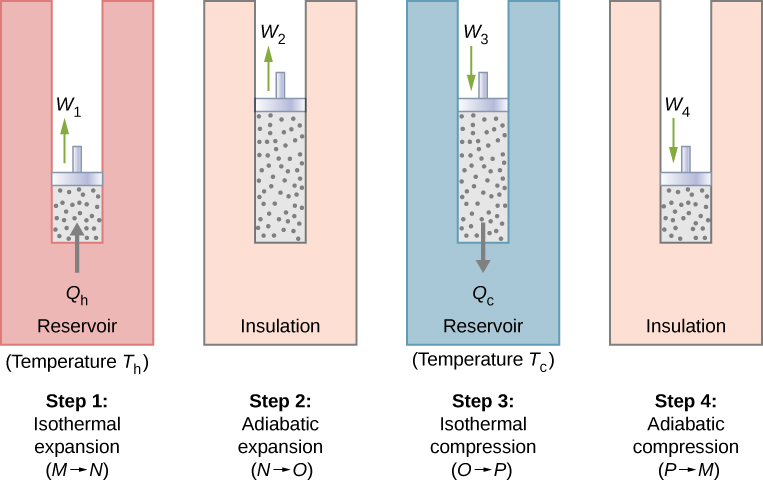
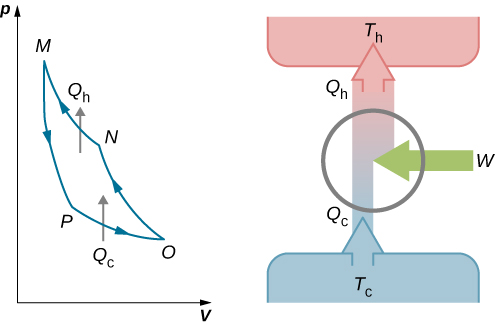
1. Heat engines
Heat engines work via the circulation of a working substance from a hot reservoir to a cold reservoir and the transformation of a part of the heat to work.
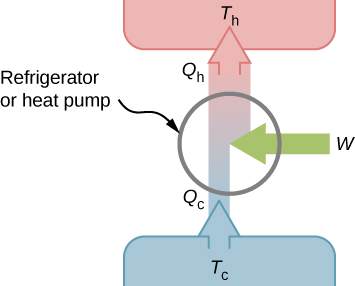
2. Refrigerators

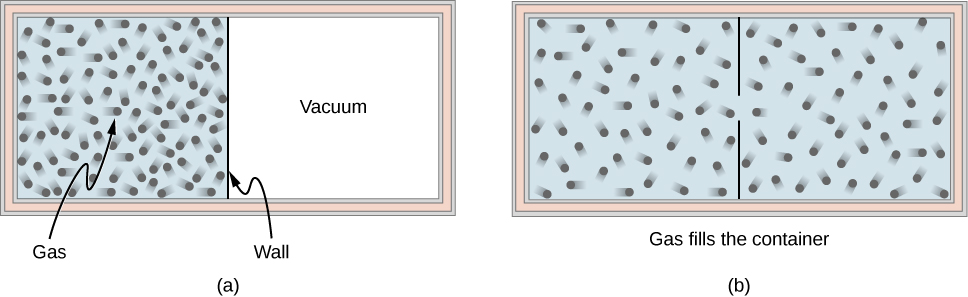
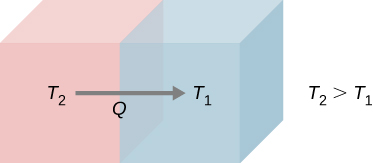
3. Second law of thermodynamics
4. Carnot's cycle